
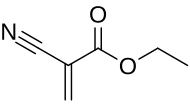
Ethyl cyanoacrylate
| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl 2-cyanoprop-2-enoate | |
| Other names
Ethyl 2-cyanoacrylate; ECA; Ethyl alpha-cyanoacrylate; 910EM; ace-ee; CN2; CN4; Cemedine 3000rs; Krazy glue; KH502; Permabond 105 : Permabond 200; Super glue; Pro grip 4000; TK 200; TK 201; Cyanolite 201; Cyanacrine; Cyano-Veneer
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.027.628 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1993 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H7NO2 | |
| Molar mass | 125.127 g·mol−1 |
| Density | 1.06 g/mL |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 54 to 56 °C (129 to 133 °F; 327 to 329 K) at 3 mmHg |
| Polymerises | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P305+P351+P338 | |
| Flash point | 83 °C (181 °F; 356 K) |
Threshold limit value (TLV)
|
0.2 ppm |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano-acrylic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names.[2] It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride.[3] ECA polymerizes rapidly in presence of moisture.

Production
Ethyl cyanoacrylate is prepared by the condensation of formaldehyde with ethyl cyanoacetate:

- NCCH2CO2C2H5 + CH2O → H2C=C(CN)CO2C2H5 + H2O
This exothermic reaction affords the polymer, which is subsequently sintered, thermally "cracked" to give the monomer. Alternatively, it can be prepared by the ethoxycarbonylation of cyanoacetylene.[2]

Applications
Ethyl cyanoacrylate is used for gluing.

In forensics, cyanoacrylate ester has excellent non-destructive impressioning abilities, which are especially important when lifting fingerprints from delicate evidence items, or when the prints could not be lifted using traditional means such as fingerprinting powder. The procedure involves heating the acrylate in a sealed chamber. Its fumes then react with deposited proteins that form into a white, stable, and clear print outlines. The resulting prints could be used 'as is' or enhanced further by staining them with darker pigments.[4][5] Liquid bandage systems use the less toxic n-butyl and octyl cyanoacrylates.

Safety
In the U.S., the threshold limit value for ECA is 0.2 ppm. It is a strong irritant to the lungs and eyes.

See also
References
- ^ GHS: Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b Ohara, Takashi; Sato, Takahisa; Shimizu, Noboru; Prescher, Günter; Schwind, Helmut; Weiberg, Otto; Marten, Klaus; Greim, Helmut; Shaffer, Timothy D.; Nandi, Partha (2020). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–21. doi:10.1002/14356007.a01_161.pub4. ISBN 9783527303854.
- ^ "Cyanoacrylate Technical Data". palmlabsadhesives.com. Archived from the original on 3 June 2009. Retrieved 12 January 2022.
- ^ Bumbrah, Gurvinder Singh (2017). "Cyanoacrylate fuming method for detection of latent fingermarks: a review". Egyptian Journal of Forensic Sciences. 7 (1): 4. doi:10.1186/s41935-017-0009-7. PMC 5514188. PMID 28781896.
- ^ Mutter, Nicole; Deacon, Paul; Farrugia, Kevin J. (2018-11-30). "The effect of cyanoacrylate fuming on subsequent protein stain enhancement of fingermarks in blood". Journal of Forensic Identification. 68 (4): 545–556. S2CID 104335657.
See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !
