
ORF7a
| Betacoronavirus NS7A protein | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of the SARS-coronavirus ORF7a accessory protein | |||||||||
| Identifiers | |||||||||
| Symbol | bCoV_NS7A | ||||||||
| Pfam | PF08779 | ||||||||
| InterPro | IPR014888 | ||||||||
| |||||||||
ORF7a (also known by several other names, including SARS coronavirus X4, SARS-X4, ORF7a, or U122)[1] is a gene found in coronaviruses of the Betacoronavirus genus. It expresses the Betacoronavirus NS7A protein, a type I transmembrane protein with an immunoglobulin-like protein domain. It was first discovered in SARS-CoV, the virus that causes severe acute respiratory syndrome (SARS).[2] The homolog in SARS-CoV-2, the virus that causes COVID-19, has about 85% sequence identity to the SARS-CoV protein.[3]

Function
A number of possible functions for the ORF7a protein have been described. The primary function is thought to be immunomodulation and interferon antagonism. The protein is not essential for viral replication.[1]

Viral protein interactions
Studies in SARS-CoV suggest that the protein forms protein-protein interactions with spike protein and ORF3a, and is present in mature virions, making it a minor viral structural protein.[1][4] It is unclear if this occurs in SARS-CoV-2.[5] It may have a role in viral assembly.[1]

Host effects
A number of interactions with host proteins and effects on host cell processes have been described. The SARS-CoV ORF7a protein has been reported to have binding activity to integrin I domains.[6]

It has also been reported to induce apoptosis via a caspase dependent pathway.[1][7] Also, it contains a motif which has been demonstrated to mediate COPII dependent transport out of the endoplasmic reticulum, and the protein is targeted to the Golgi apparatus.[8]

In SARS-CoV-2, ORF7a protein has been described as an effective interferon antagonist.[3] The SARS-CoV-2 protein may have immunomodulatory effects through interaction with monocytes.[5]

Structure
The ORF7a protein is a transmembrane protein with 121 amino acid residues in SARS-CoV-2[5] and 122 in SARS-CoV.[2] It is a type I transmembrane protein with an N-terminal signal peptide, an ectodomain that has an immunoglobulin fold, and a C-terminal endoplasmic reticulum retention signal sequence.[5][6][1] The structure contains seven beta strands which form two beta sheets, arranged in a beta sandwich.[2] Most of the sequence differences between SARS-CoV and SARS-CoV-2 occur in the Ig-like ectodomain and may produce differences in protein-protein interactions.[5]

Post-translational modifications
The SARS-CoV-2 ORF7a protein has been reported to be post-translationally modified by ubiquitination. Polyubiquitin chains attached to lysine 119 may be related to the protein's reported interferon antagonism.[3][9]

Expression and localization
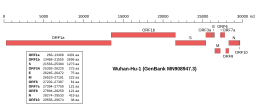 Genomic organisation of isolate Wuhan-Hu-1, the earliest sequenced sample of SARS-CoV-2, indicating the location of ORF7a | |
| NCBI genome ID | 86693 |
|---|---|
| Genome size | 29,903 bases |
| Year of completion | 2020 |
| Genome browser (UCSC) | |
Along with the genes for other viral accessory proteins, the ORF7a gene is located near those encoding the viral structural proteins, at the 5' end of the coronavirus RNA genome.[3] ORF7a is an overlapping gene that overlaps ORF7b.[10] In SARS-CoV, subcellular localization to the endoplasmic reticulum, Golgi apparatus, and ERGIC has been reported,[1] with similar Golgi localization described for SARS-CoV-2.[11]

Evolution

It is thought that ORF8 in SARS-CoV-2, which encodes a protein with a similar Ig-like fold, may be a paralog of ORF7a that originated through gene duplication,[13][14] though some bioinformatics analyses suggest the similarity may be too low to support duplication, which is relatively uncommon in viruses.[15] Immunoglobulin domains are uncommon in coronaviruses; other than the subset of betacoronaviruses with ORF8 and ORF7a, only a small number of bat alphacoronaviruses have been identified as containing likely Ig domains, while they are absent from gammacoronaviruses and deltacoronaviruses.[16][14] The beta and alpha Ig domains may be independent acquisitions, where ORF8 and ORF7a may have been acquired from host proteins.[16]

Many SARS-CoV-2 genomes have been sequenced throughout the COVID-19 pandemic and a number of variations have been reported, including deletion mutations,[17] nonsense mutations (introducing a premature stop codon and truncating the protein),[18] and at least one gene fusion.[19]

References
- ^ a b c d e f g Liu, DX; Fung, TS; Chong, KK; Shukla, A; Hilgenfeld, R (September 2014). "Accessory proteins of SARS-CoV and other coronaviruses". Antiviral Research. 109: 97–109. doi:10.1016/j.antiviral.2014.06.013. PMC 7113789. PMID 24995382.
- ^ a b c Nelson CA, Pekosz A, Lee CA, Diamond MS, Fremont DH (2005). "Structure and intracellular targeting of the SARS-coronavirus Orf7a accessory protein". Structure. 13 (1): 75–85. doi:10.1016/j.str.2004.10.010. PMC 7125549. PMID 15642263.
- ^ a b c d Redondo, Natalia; Zaldívar-López, Sara; Garrido, Juan J.; Montoya, Maria (7 July 2021). "SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns". Frontiers in Immunology. 12: 708264. doi:10.3389/fimmu.2021.708264. hdl:10261/249329. PMC 8293742. PMID 34305949.
- ^ Huang, Cheng; Ito, Naoto; Tseng, Chien-Te K.; Makino, Shinji (August 2006). "Severe Acute Respiratory Syndrome Coronavirus 7a Accessory Protein Is a Viral Structural Protein". Journal of Virology. 80 (15): 7287–7294. doi:10.1128/JVI.00414-06. PMC 1563709. PMID 16840309.
- ^ a b c d e f Zhou, Ziliang; Huang, Chunliu; Zhou, Zhechong; Huang, Zhaoxia; Su, Lili; Kang, Sisi; Chen, Xiaoxue; Chen, Qiuyue; He, Suhua; Rong, Xia; Xiao, Fei; Chen, Jun; Chen, Shoudeng (March 2021). "Structural insight reveals SARS-CoV-2 ORF7a as an immunomodulating factor for human CD14+ monocytes". iScience. 24 (3): 102187. Bibcode:2021iSci...24j2187Z. doi:10.1016/j.isci.2021.102187. PMC 7879101. PMID 33615195.
- ^ a b Hänel K, Stangler T, Stoldt M, Willbold D (May 2006). "Solution structure of the X4 protein coded by the SARS related coronavirus reveals an immunoglobulin like fold and suggests a binding activity to integrin I domains". J. Biomed. Sci. 13 (3): 281–93. doi:10.1007/s11373-005-9043-9. PMC 7089389. PMID 16328780.
- ^ Satija N, Lal SK (2007). "The molecular biology of SARS coronavirus". Ann N Y Acad Sci. 1102 (1): 26–38. Bibcode:2007NYASA1102...26S. doi:10.1196/annals.1408.002. PMC 7168024. PMID 17470909.
- ^ Pekosz A, Schaecher SR, Diamond MS, Fremont DH, Sims AC, Baric RS (2006). "Structure, expression, and intracellular localization of the SARS-CoV accessory proteins 7a and 7b". Adv Exp Med Biol. Advances in Experimental Medicine and Biology. 581: 115–20. doi:10.1007/978-0-387-33012-9_20. ISBN 978-0-387-26202-4. PMC 7123408. PMID 17037516.
- ^ Cao, Zengguo; Xia, Hongjie; Rajsbaum, Ricardo; Xia, Xianzhu; Wang, Hualei; Shi, Pei-Yong (March 2021). "Ubiquitination of SARS-CoV-2 ORF7a promotes antagonism of interferon response". Cellular & Molecular Immunology. 18 (3): 746–748. doi:10.1038/s41423-020-00603-6. PMC 7815971. PMID 33473190.
- ^ Pekosz, Andrew; Schaecher, Scott R.; Diamond, Michael S.; Fremont, Daved H.; Sims, Amy C.; Baric, Ralph S. (2006). "Structure, Expression, and Intracellular Localization of the SARS-CoV Accessory Proteins 7a and 7b". The Nidoviruses. Advances in Experimental Medicine and Biology. 581: 115–120. doi:10.1007/978-0-387-33012-9_20. ISBN 978-0-387-26202-4. PMC 7123408. PMID 17037516.
- ^ Zhang, Jing; Cruz-cosme, Ruth; Zhuang, Meng-Wei; Liu, Dongxiao; Liu, Yuan; Teng, Shaolei; Wang, Pei-Hui; Tang, Qiyi (December 2020). "A systemic and molecular study of subcellular localization of SARS-CoV-2 proteins". Signal Transduction and Targeted Therapy. 5 (1): 269. doi:10.1038/s41392-020-00372-8. PMC 7670843. PMID 33203855.
- ^ Flower, Thomas G.; Buffalo, Cosmo Z.; Hooy, Richard M.; Allaire, Marc; Ren, Xuefeng; Hurley, James H. (12 January 2021). "Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein". Proceedings of the National Academy of Sciences. 118 (2): e2021785118. doi:10.1073/pnas.2021785118. PMC 7812859. PMID 33361333.
- ^ Mariano, Giuseppina; Farthing, Rebecca J.; Lale-Farjat, Shamar L. M.; Bergeron, Julien R. C. (17 December 2020). "Structural Characterization of SARS-CoV-2: Where We Are, and Where We Need to Be". Frontiers in Molecular Biosciences. 7: 605236. doi:10.3389/fmolb.2020.605236. PMC 7773825. PMID 33392262.
- ^ a b Neches, Russell Y.; Kyrpides, Nikos C.; Ouzounis, Christos A. (23 February 2021). "Atypical Divergence of SARS-CoV-2 Orf8 from Orf7a within the Coronavirus Lineage Suggests Potential Stealthy Viral Strategies in Immune Evasion". mBio. 12 (1). doi:10.1128/mBio.03014-20. PMC 7845636. PMID 33468697.
- ^ Pereira, Filipe (November 2020). "Evolutionary dynamics of the SARS-CoV-2 ORF8 accessory gene". Infection, Genetics and Evolution. 85: 104525. doi:10.1016/j.meegid.2020.104525. PMC 7467077. PMID 32890763.
- ^ a b Tan, Yongjun; Schneider, Theresa; Leong, Matthew; Aravind, L.; Zhang, Dapeng (30 June 2020). "Novel Immunoglobulin Domain Proteins Provide Insights into Evolution and Pathogenesis of SARS-CoV-2-Related Viruses". mBio. 11 (3). doi:10.1128/mBio.00760-20. PMC 7267882. PMID 32471829.
- ^ Holland, LaRinda A.; Kaelin, Emily A.; Maqsood, Rabia; Estifanos, Bereket; Wu, Lily I.; Varsani, Arvind; Halden, Rolf U.; Hogue, Brenda G.; Scotch, Matthew; Lim, Efrem S. (July 2020). "An 81-Nucleotide Deletion in SARS-CoV-2 ORF7a Identified from Sentinel Surveillance in Arizona (January to March 2020)". Journal of Virology. 94 (14): e00711-20. doi:10.1128/JVI.00711-20. PMC 7343219. PMID 32357959.
- ^ Nemudryi, Artem; Nemudraia, Anna; Wiegand, Tanner; Nichols, Joseph; Snyder, Deann T.; Hedges, Jodi F.; Cicha, Calvin; Lee, Helen; Vanderwood, Karl K.; Bimczok, Diane; Jutila, Mark A.; Wiedenheft, Blake (June 2021). "SARS-CoV-2 genomic surveillance identifies naturally occurring truncation of ORF7a that limits immune suppression". Cell Reports. 35 (9): 109197. doi:10.1016/j.celrep.2021.109197. PMC 8118641. PMID 34043946.
- ^ Addetia, Amin; Xie, Hong; Roychoudhury, Pavitra; Shrestha, Lasata; Loprieno, Michelle; Huang, Meei-Li; Jerome, Keith R.; Greninger, Alexander L. (August 2020). "Identification of multiple large deletions in ORF7a resulting in in-frame gene fusions in clinical SARS-CoV-2 isolates". Journal of Clinical Virology. 129: 104523. doi:10.1016/j.jcv.2020.104523. PMC 7309833. PMID 32623351.
See what we do next...
OR
By submitting your email or phone number, you're giving mschf permission to send you email and/or recurring marketing texts. Data rates may apply. Text stop to cancel, help for help.
Success: You're subscribed now !

